The Wang Laboratory (yLab) synthetically creates new materials with exciting new properties and functions. Most recent examples include the discovery and development of (1) quantum defects (a.k.a., “sp3 quantum defects” or “organic color-centers”), (2) fluorescent ultrashort nanotubes (FUNs), (3) tube-in-a-tube semiconductors (Tube^2), (4) photoactuated polymer pens, and (5) metatextiles. We also develop instrumentation, methodologies, and platform technologies to enable single defect spectroscopy, molecular printing, and clean carbon technology as well as dynamic gating of infrared radiation in a textile. The new materials, chemistry, and tools we are developing, often through fruitful collaborations with others, directly address fundamental questions in energy, biomedical, and quantum technologies. For example, how are electrons, excitons, phonons, and spin coupled at atomic defects? How does a defect-trapped exciton respond to local chemical events? What if we could shrink a pH meter all the way down to the nanometer scale or even the size of a single atomic defect? What are the fingerprints of a disease and how can we detect cancer at the earliest possible stage? What if we could create a cloth that adapts to heat and can be manufactured on a large scale? …
1. Quantum Defects. Defects can rule the properties of a crystal. This effect is particularly intriguing in atom-thick low-dimensional materials, such as single-walled 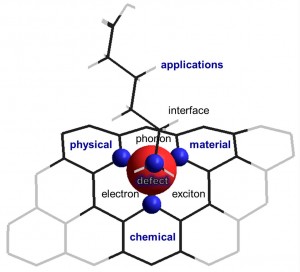 carbon nanotubes (SWCNTs) and graphene, where new chemistry and physics may arise due to strong coupling of electrons, excitons, phonons, and spin at the atomic defects. Since the accidental discovery of quantum defects (a.k.a., organic color centers) in our laboratory, we, collaborating with many fantastic colleagues around the world, have been busy in synthetically creating this entirely new family of quantum emitters and exploring these chemically-tailored, fluorescent quantum defects as a new toolkit for materials engineering, brightening of dark excitons, synthesizing quantum emitters and single photon sources, and probing chemical events that could be otherwise difficult to capture.
carbon nanotubes (SWCNTs) and graphene, where new chemistry and physics may arise due to strong coupling of electrons, excitons, phonons, and spin at the atomic defects. Since the accidental discovery of quantum defects (a.k.a., organic color centers) in our laboratory, we, collaborating with many fantastic colleagues around the world, have been busy in synthetically creating this entirely new family of quantum emitters and exploring these chemically-tailored, fluorescent quantum defects as a new toolkit for materials engineering, brightening of dark excitons, synthesizing quantum emitters and single photon sources, and probing chemical events that could be otherwise difficult to capture.
Selected Publications:
“Engineering Defects with DNA.” Wang, Y. H. Science 2022, 377, 473-474.
“Quantum Defects: What pairs with the aryl group when breaking a C=C bond on sp2 carbon lattices?” Wang, P.; Fortner, J.; Luo, H.; Kłos, J.; Wu, X.; Qu, H.; Chen, F.; Li, Y.; Wang, Y.H. J. Am. Chem. Soc. 2022, 144, 29, 13234-13241.
“Reconfiguring Organic Color Centers on the sp2 Carbon Lattice of Single-Walled Carbon Nanotubes.” Qu, H.; Wu, X.; Fortner, J.; Kim, M.; Wang, P.; Wang, Y.H.* ACS Nano 2022, 16, 2, 2077–2087.
“Formation of Organic Color Centers in Air-Suspended Carbon Nanotubes Using Vapor-Phase Reaction” D. Kozawa,* X. Wu, A. Ishii, J. Fortner, K. Otsuka, R. Xiang, T. Inoue, S. Maruyama, Y. Wang, Y. K. Kato*. Nature Communications 2022, 13: 2814.
“Single-defect spectroscopy in the shortwave infrared.” Wu, X.J.; Kim, M.J.; Qu, H.; Wang, Y.H.* Nature Communications 2019, 10: 2672.
“Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects.” Brozena, A.; Kim, M.J.; Powell, L. R.; Wang, Y. H.*. Nature Reviews Chemistry 2019, 3, 375–392. DOI: 10.1038/s41570-019-0103-5.
“Probing Trions at Chemically Tailored Trapping Defects.” Kwon, H.J.; Kim, M.; Nutz, M; Hartmann, N.F.; Perrin, V.; Meany, B.; Hofmann, M. S.; Clark, C.W.; Htoon, H.; Doorn, S.K.; Högele, A.; Wang, Y.H.* ACS Central Science 2019, 5, 1786-1794. (Supplementary Journal Cover)
“Molecularly tunable fluorescent quantum defects.” Kwon, H.; Furmanchuk, A.; Kim, M.; Meany, B.; Guo, Y.; Schatz, G. C.*; Wang, Y.H.* J. Am. Chem. Soc. 2016, 138, 6878-6885.
“Optical probing of local pH and temperature in complex fluids with covalently functionalized, semiconducting carbon nanotubes.” Kwon, H.‡, Kim, M.‡, Meany, B.; Piao, Y.; Powell, L. R.; Wang, Y.H.*. J. Phys. Chem. C 2015, 119, 3733–3739.
“Brightening of carbon nanotube photoluminescence through the incorporation of sp3 defects.” Piao, Y. M.; Meany, B.; Powell, L. R.; Valley, N.; Kwon, H.; Schatz, G. C.; Wang, Y. H.* Nature Chemistry 2013, 5, 840-845.
2. Tube^2. Atom-thick materials, such as SWCNTs and graphene, are prone to chemical attacks because all of the constituent atoms are exposed. To overcome this materials limitation, we are synthesizing a class of tube-in-a-tube (Tube^2) semiconductors that is
 equivalent to a SWCNT hermetically sealed by a chemically tailored outer wall. We have created Tube^2 prototypes from double-walled carbon nanotubes through wall-selective covalent chemistry and most recently, by coating SWCNTs with BN to form one-dimensional van der Waals heterostructures. This tube-in-a-tube concept allows us to address a series of fundamental questions central to nanomaterials chemistry. For example, how are the electronic properties of a semiconducting nanostructure affected by its chemical environment? What if the environmental effects could be controlled or completely eliminated by protecting the inner tube with the outer wall? Will electrical transport in such a “double wall” structure become immune to chemical perturbations by oxygen and water? Will it fluoresce brightly?
equivalent to a SWCNT hermetically sealed by a chemically tailored outer wall. We have created Tube^2 prototypes from double-walled carbon nanotubes through wall-selective covalent chemistry and most recently, by coating SWCNTs with BN to form one-dimensional van der Waals heterostructures. This tube-in-a-tube concept allows us to address a series of fundamental questions central to nanomaterials chemistry. For example, how are the electronic properties of a semiconducting nanostructure affected by its chemical environment? What if the environmental effects could be controlled or completely eliminated by protecting the inner tube with the outer wall? Will electrical transport in such a “double wall” structure become immune to chemical perturbations by oxygen and water? Will it fluoresce brightly?
Selected Publications:
“Parallel field-effect nanosensors detect trace biomarkers instantly at physiological high ionic strength conditions.” Barnes, B.; Wang, P.; Wang, Y. H. ACS Sensors 2022 in press (Journal Cover).
“Van der Waals SWCNT@BN Heterostructures Synthesized from Solution-Processed Chirality-Pure Single-Wall Carbon Nanotubes.” Chiyu Zhang, Jacob Fortner, Peng Wang, Jeffrey A. Fagan, Shuhui Wang, Ming Liu, Shigeo Maruyama, YuHuang Wang*. ACS Nano 2022, 16, 18630-18636.
Barnes, B.; Brozena, A.; Wang, Y.H. The Biochemist. 2019, 41 (4), page 10-13 (A special issue celebrating Elements in Biochemistry from the Biochemical Society; invited perspective article).
“Laser lithography of a tube-in-a-tube nanostructure.” Ng, A.L.; Piao, Y.; Wang, Y.H.* ACS Nano 2017 (ASAP). DOI: 10.1021/acsnano.7b00624.
“Chemical gating of a synthetic tube-in-a-tube semiconductor.” Ng, A.L.; Chen, C.-F.; Kwon, H.; Peng, Z.; Lee, C.S.; Wang, Y.H.* J. Am. Chem. Soc., 2017, ASAP.
“Covalently functionalized double-walled carbon nanotubes combine high sensitivity and selectivity in the electrical detection of small molecules.” Huang, J.; Ng, A.; Piao, Y. M.; Chen, C. F.; Green, A.; Hersam, M. C.; Lee, C.; Wang, Y. H.* J. Am. Chem. Soc. 2013, 135, 2306-2312.
“Optical and electrical properties of inner tubes in outer wall-selectively functionalized double-wall carbon nanotubes.” Piao, Y. M.; Chen, C. F.; Green, A. A.; Kwon, H.; Hersam, M. C.; Lee, C. S.; Schatz, G. C.; Wang, Y. H.* J. Phys. Chem. Lett. 2011, 2, 1577-1582.
“Double-walled carbon nanotubes: challenges and opportunities.” Shen, C.; Brozena, A. H.; Wang Y. H.* Nanoscale 2011, 3, 503 – 518. (invited review article).
“Outer wall selectively oxidized, water-soluble double-walled carbon nanotubes.” Brozena, A.H.; Moskowitz, J.♯; Shao, B.♯; Deng, S. L.; Liao, H. W.; Gaskell K. J.; Wang, Y. H.* J. Am. Chem. Soc. 2010, 132, 3932-3938.
3. Fluorescent Ultrashort Nanotubes: Just for FUNs
Ultrashort single-walled carbon nanotubes that fluoresce brightly in the shortwave infrared could open exciting opportunities in high-resolution bioimaging and sensing. However, this material remains largely unexplored due to the synthetic challenge. We are developing chemical approaches to synthesize and characterize FUNs and apply them for various applications in collaboration with others worldwide.
Selected Publications:
“Fluorescent Ultrashort Nanotubes from Defect-Induced Chemical Cutting.” Li, Yunfeng; Wu, Xiaojian; Kim, Mijin; Qu, Haoran; Fortner, Jacob; Wang, YuHuang*. Chemistry of Materials 2019, 31, 4536-4544.
“Ultrashort carbon nanotubes that fluoresce brightly in the near infrared.” Danné, N.; Kim, M.; Godin, A.G.; Kwon, H.; Gao, Z.H.; Wu, J.X.; Hartmann, N.; Doorn, S.K.; Lounis, B.; Wang, Y.H.; Cognet, L.* ACS Nano 2018, 12 (6), 6059–6065
4. Question Y
We like to ask impossible questions, and we are always seeking the wow moments. Stay tuned.
Selected Publications:
“Superacid-Surfactant Exchange: Enabling Non-destructive Dispersion of Full-length Carbon Nanotubes in Water.” Wang, P.; Kim, M.; Peng, Z.; Sun, C.-F.; Lieberman, A.; Mok, J.; Wang, Y. H.* ACS Nano 2017, 11 (9), pp 9231–9238.
“Dynamic Gating of Infrared Radiation in a Textile.” Zhang, X.; Yu, S.J.; Xu, B.B.; Li, M.; Peng, Z. W.; Wang, Y.; Deng, S.L.; Wu, X.J.; Wu, Z.P.; Ouyang, M.*; Wang, Y. H.* Science 2019, 363, 619-623. Read a free copy of Reprint & Full Text courtesy of Science. This article is widely reported by news media including C&EN, New Scientist, MIT Technology Review, Scientific American, The Washington Post, BBC, The Times, and many others
“Detection of ovarian cancer via the spectral fingerprinting of quantum-defect-modified carbon nanotubes in serum by machine learning” Kim, M.; Chen, C.; Wang, P.; Yang, Y.; Mulvey, J.; Wun, C.; Antman-Passig, M.; Luo, H.; Cho, S.; Long-Roche, K.; Ramanathan, L. V.; Jagota, A.; Zheng, M.; Wang, Y.; Heller, D. A.* Nature Biomedical Engineering 2022, 6, 267–275 (Journal Cover).
5. Nanoelectrode Networks for Energy Storage and Harvesting. We are researching novel chemical methods and nanofabrication approaches to integrate CNTs for a wide range of basic and applied research, including lithium-ion batteries and solar cells. The projects in our lab currently focus on studying electrical transport through nanotube interfaces and synthesizing heterogeneous nanostructures that may mechanically self-heal and are electrochemically reversible as the anode of lithium-ion batteries. The remarkable electron-accepting capabilities and charge transport properties of SWCNTs have also suggested new possibilities for overcoming the efficiency bottleneck currently facing several next-generation solar cells. CNTs can significantly improve the performance of organic photovoltaic cells and the photoconversion efficiency of TiO2-based Grätzel cells by effectively collecting and shuttling the electrons injected from light harvesting components (e.g., porphyrins) or charge separation centers (e.g., TiO2 nanoparticles). The efficient electron transport also facilitates charge separation and prevents charge recombination, which may improve photoconversion efficiency.
Selected Publications:
“Probing the Electrical Double Layer by operando X-ray Photoelectron Spectroscopy through a Carbon Nanotube-Strengthened Graphene Window.” Wang, P.+; Li, Y.+; Wang, L.; Klos, J.; Peng, Z.; Kim, N.; Bluhm, H.; Gaskell, K.; Liu, P.; Lee, S.B.; Eichhorn, B.*; Wang, Y.* EcoMat 2020; 2:e12023. https://doi.org/10.1002/eom2.12023. Also check out the force field deposited at Zenodo.
“Concentrated Electrolytes Stabilize Bismuth-Based Potassium-Ion Batteries.” Zhang, R.; Bao, J.Z.; Wang, Y.H.; Sun, C.-F.* Chemical Science 2018, 9, 6193-6198.
“Li3PO4 matrix enables long cycle life and high energy efficiency bismuth-based battery.” Sun, C.-F.; Hu, J.K.; Wang, P.; Cheng, X.-Y.; Lee, S. B.*; Wang, Y.H.* Nano Letters 2016 (accepted, article ASAP).
“Interfacial oxygen stabilizes silicon anodes.” Sun, C.-F.; Zhu, H.-L.; Okada, M.; Gaskell, K.; Inoue, Y.; Hu, L.; Wang, Y. H.* Nano Letters 2015, 15, 703-708.
“A beaded-string Silicon Anode.” Sun, C.F.; Karki, K.; Jia, Z.; Liao, H.; Zhang, Y.; Li, T.*; Qi, Y.*; Cumings, J.*; Rubloff, G. W.; Wang, Y. H.* ACS Nano 2013, 7, 2717-2724.
“Nanofabrication beyond electronics.” Wang, Y. H.*; Mirkin, C. A.; Park, S.-J. ACS Nano 2009, 3, 1049-1056 (invited perspective).
6. Ultra-selective Carbon Network Chemistry. Single-walled carbon nanotubes (SWCNTs) are a class of nanostructured materials that combine remarkable electrical, mechanical, thermal, and optical properties all in one. Unlike other nanostructures, such as quantum dots, SWCNTs are (in a sense) molecules. Their fascinating properties are not simply a result of size effect, but originate from an intertwining of the one-dimensional confinement of electronic states with the incredible versatility of how the carbon-carbon bonds are arranged within the tubular network. Within a small diameter range (0.4–2 nm), an SWCNT can have over 150 possible chiral structures, each uniquely indexed by a pair of integers (n,m). For two SWCNTs that differ in diameter by less than 0.01 nm (e.g., [10,10] vs. [11,9]), one has an electrical conductivity rivaling copper, while the other is predicted to be a semiconductor with interesting optical properties. This dramatic change of properties with a subtle difference in structure gives rise to a vast new realm of chemistry and physics. However, all current synthetic methods for making SWCNTs yield a complex mixture of all types of nanotubes, and moreover, SWCNTs are virtually insoluble in any conventional solvent. These two problems have held back a host of potential applications and the full establishment of this new branch of molecular science, but they also have persistently challenged some of the limits of nanoscience and nanotechnology from the perspective of chemical synthesis, catalysis, complex mixture separation, metrology, and nanostructure manipulation. To meet some of these challenges, we are developing new materials strategies to eliminate the insolubility problem facing SWCNTs and new methods to effect the separation and manipulation on the single chirality level.
Selected Publications:
“Optically triggered melting of DNA on individual semiconducting carbon nanotubes.” Wang, C.-Y.; Meany, B.; Wang, Y.H.* Angew. Chem. Int. Ed., 2017, 56, 9326-9330.
“Achieving ultrahigh concentrations of fluorescent single-walled carbon nanotubes using small-molecule viscosity modifiers.” Leed, J. D.; Fourkas, J. T.*; Wang, Y. H.* Small 2013, 9, 241-247.
“Acyclic cucurbit[n]uril molecular clips selectively solubilize single-walled carbon nanotubes in water.” Shen, C.; Da, M.; Meany, B.; Issacs, L.*; Wang, Y. H.* J. Am. Chem. Soc. 2012, 134, 7254−7257.
“Outerwall selective alkylcarboxylation and enrichment of double-walled carbon nanotubes.” Deng, S.; Piao, Y.-M.; Brozena, A. H.; Wang Y. H.* J. Mater. Chem. 2011, 21, 18568-18574.
“Diameter-dependent, progressive alkylcarboxylation of single-walled carbon nanotubes.” Deng, S.; Brozena, A. H.; Zhang, Y.; Piao, Y.-M.; Wang, Y. H.* Chem. Comm. 2011, 47, 758-760.
“Confined propagation of covalent chemical reactions on single-walled carbon nanotubes.” Deng, S.; Zhang, Y.; Brozena, A. H.; Mayes, M. L.; Banerjee, P.;Chiou, W. A.; Rubloff, G. W.; Schatz, G. C.; Wang Y. H.* Nature Communications 2011 2:382 doi: 10.1038/ncomms1384.
7. “Seeded Crystallization” of Nanostructures: From Discrete Nanoparticles to Organized Solids. A group of atoms is often not functional until they are made into a molecule, and depending on how the atomic building blocks are bonded, their collective properties may be totally different. Will nanostructures behave similarly to atomic building blocks? The ability to assemble isolated nanostructures into ordered solids and functional networks may open a new world. This capability will also be ultimately required for the realization of many potential applications based on nanomaterials, such as nanoelectronics, quantum wires, photonic crystals, and solar cells. Unlike molecules or atoms, little is known about the fundamental principles that govern the self-assembly and directed assembly of nanostructures. We will approach this problem by making an analogy to crystallization. For example, how does self-assembly nucleate on a surface? Can one plant a “seed” to direct the subsequent assembly? Is it possible to introduce long-range order into a self-assembled system by planting a series of “seeds” that are correlated in space and symmetry? Will this bottom-up approach afford both a packing density far beyond the resolution of lithography and long-range registry that is typically missing in self-assembled systems?
