The Vedernikov
Group
![]()
Dr. Andrei N.
Vedernikov
|
|
Professor
Inorganic, Organometallic, Organic, and Computational Chemistry Ph.D. 1986,
Kazan State
University, Russia Department of Chemistry &
Biochemistry |
| Research Interests Development of new bond breaking (C-H, B-C, O=O) and bond making (C-O, C-N, C-C, B-C) processes, manipulation with kinetically inert molecules (alkanes including methane, dioxygen etc.). Aerobic organoplatinum(II) and organopalladium(II) chemistry. New synthetic strategies. Experimental and calculational (ab initio, DFT) organotransition metal chemistry. Mechanistic and theoretical study of organometallic reactions, including small molecule activation. Design of new ligands and catalysts for organic reactions.
Aerobic Organoplatinum(II)
Chemistry
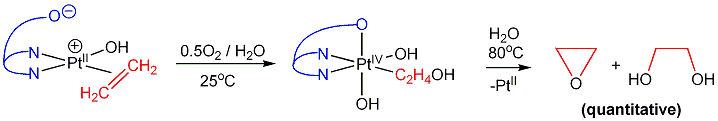
b) (dpms)PtII(ethene)OH complex reacts cleanly with O2 in water at room temperature via 2-hydroxyethyl PtII intermediate to produce (dpms)PtIV(C2H4OH)(OH)2 complex. The latter eliminates ethylene oxide at 80oC:
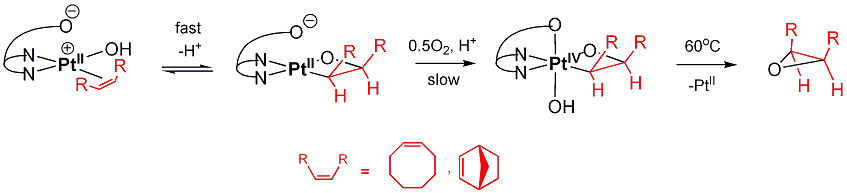
c) Analogous cycloolefin complexes produce readily PtII- and PtIV-oxetanes. The latter eliminate corresponding epoxides:
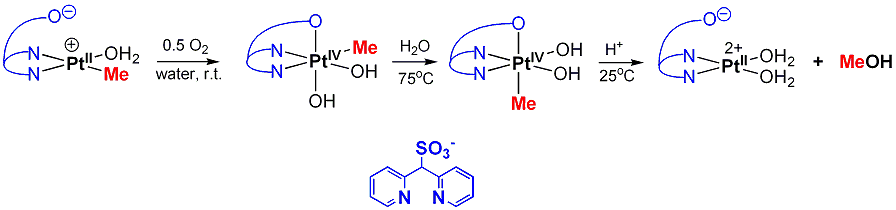
Available data indicates that anionic intermediates (dpms)PtIIR(X)- are responsible for dioxygen activation.
New
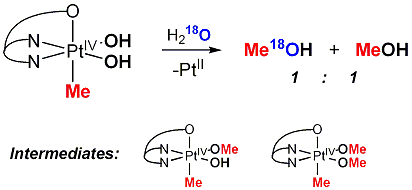
Mechanisms of C(sp3)-O Reductive Elimination
from PtIV
b) In certain cases an unprecedented for PtIV direct intramolecular C-O elimination is observed (see elimination of epoxides from PtIV oxetanes above). This reaction is fundamentally different from SN2 mechanism typical for PtIVMe complexes and is also stereospecific. The order of reactivity of PtIV alkyls in direct C-O elimination is as follows: 2o alkyl > 1o alkyl > Me. The opposite order of reactivity is observed in SN2 reactions. The observations above may be valuable for designing Pt-mediated selective hydrocarbon functionalization reactions in hydroxylic solvents including water.
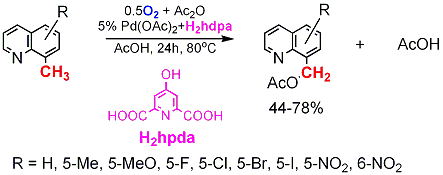
Catalytic Aerobic Organopalladium(II)
Chemistry
The catalytic oxidation reaction is selective; a number of functional groups are tolerated. One of the suggested reaction mechanisms involves direct aerobic oxidation of alkypalladium(II) species, PdII(Hpda)(N-C), to produce reactive alkylpalladium(IV) intermediates. Financial support to this work from the Donors of the American Chemical Society Petroleum Research Fund (PRF#42307-AC3), National Science Foundation (CHE-0614798), the US-Israel Binational Science Foundation and the Center for Catalytic Hydrocarbon Functionalization (CCHF), an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (Award Number DE-SC0001298) is gratefully acknowledged. |
|
University
of Maryland
091 Chemistry Building
Department of Chemistry & Biochemistry, Rm 2353
College Park, MD 20742
Phone: (301) 405-2784
Fax: (301) 314-9121
Last updated:
09 February 2023