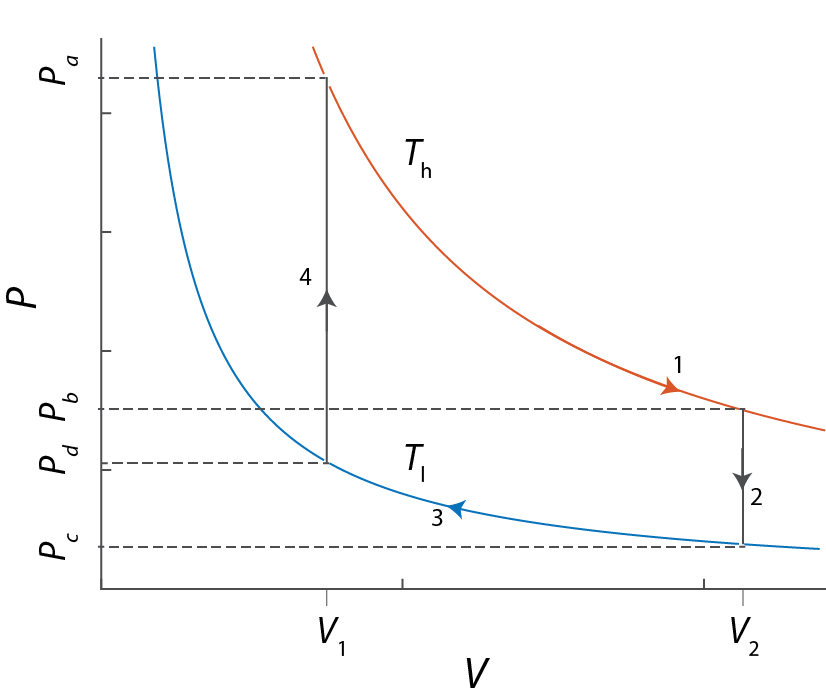
-
Reversible expansion and compression
- The work associated with steps 1 and 3 is $dw=-\int PdV$ where $P$ is the instantaneous pressure of the gas. For an ideal gas, this is $dw = - NRT\ln(V_{final}-V_{initial})$.
- For steps 2 and 4, since the volume doesn't change, $w=0$. Then, the First Law tells us that $dU = \delta q$, or $q=C_{\rm V} (T_{final}-T_{initial})$. Also, since Steps 1 and 3 are isothermal, $\Delta U = 0$, so that $q=-w$.
- Irreversible expansion and compression
- heat flow for each step is formally unchanged, except that the work associated for steps 1 and 3 is different.
-
For a one-step (irreversible) expansion or compression the work is $dw=-\int P_{ext}dV = -P_{ext}\Delta V$. For the expansion step, the external pressure cannot be greater than $P_b$ (see the above diagram).
For the compression step, the external pressure has to be at least equal to $P_d$.
From the ideal gas law, $P_b=NRT_h/V_2$ and $P_d=NRT_l/V_1$.
- The following tables summarize these results (with $\Delta V=V_2-V_1$ and $\Delta T= T_h-T_l$; note that both $\Delta V$ and $\Delta T$ are positive).
Step
Reversible expansion and compression
Work
Heat
1
$-NRT_h\ln(V_2/V_1)$
$+NRT_h\ln(V_2/V_1)$
2
0
$-C_{\rm V}\Delta T$
3
$+NRT_l\ln(V_2/V_1)$
$-NRT_l\ln(V_2/V_1)$
4
0
$+C_{\rm V}\Delta T$
$\oint$
$-NR\ln(V_2/V_1)(T_h-T_l)$
$+NR\ln(V_2/V_1)(T_h-T_l)$
Step
Irreversible
Work
Heat
1
$-NRT_h\Delta V/V_2$
$+NRT_h\Delta V/V_2$
2
0
$-C_{\rm V}\Delta T$
3
$+NRT_l\Delta V/V_1$
$-NRT_1\Delta V/V_1$
4
0
$+C_{\rm V}\Delta T$
$\oint$
$-NR\Delta V\left (T_h\,/V_2-T_l\,/V_1\right )$
$+NR\Delta V\left (T_h\,/V_2-T_l\,/V_1\right )$
- If steps 1 and 3 are reversible: $\eta = (T_h-T_l)/T_h$, identical to the Carnot efficiency.
- If steps 1 and 3 are single-step: $$\eta = \frac{T_h/V_2-T_l/V_1}{T_h/V_2)}= 1-\frac{T_l}{T_h}\frac{V_2}{V_1}$$
Obviously if the temperatures and the volumes don't satisfy the inequality $T_h \gt T_l \,(V_2/V_1)$, then the efficiency will turn out to be a negative number (indicative that the whole cycle is not producing work!).