- NTERM (the number of potential surfaces involved: this can be 1, 2, or 4 here
and must be consistent with the POT subroutine). This parameter can not be changed
NVMINS: lowest vibrational level for sigma state
NVMAXS: higest vibrational level for sigma state
NVMINP: lowest vibrational level for pi state
NVMAXP: higest vibrational level for pi state
JMIN: the minimum rotational angular momenta for each 2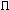 channel
channel
JMAX: the maximum rotational angular momenta for each 2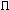 channel
channel
in each spin-orbit manifold with convention omega .le. j .le. jmax+0.5
NMIN: the minimum Hund's case (b) rotational angular momenta for the
2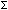 state
state
NMAX: the maximum Hund's case (b) rotational angular momenta for the
2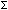 state
state
 JMIN, JMAX, NMIN, NMAX are defined separately for each vibrational level
JMIN, JMAX, NMIN, NMAX are defined separately for each vibrational level
IPERT: Each vibrational Pi level ivp may be perturbed by one sigma vibrational level IVS. This level is given by ivs=ipert(ivp) (see hisysgpi)
IGUPI: permutation inversion symmetry of 2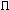 electronic state
electronic state
- IGU = 1 for gerade states
IGU = -1 for ungerade states
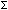 electronic state
electronic state
- IGU = 1 for gerade states
IGU = -1 for ungerade states
 for heteronuclear molecules both IGUPI and IGUSG should be +1
for heteronuclear molecules both IGUPI and IGUSG should be +1
NPARPI: number of 2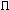 symmetry doublets
included
symmetry doublets
included
- NPAR = 2 will ensure
both lambda doublets
NPARPI = 1 just
 = 1 doublets;
= 1 doublets;
NPARPI = -1, just
 = -1
doublets
= -1
doublets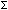 state included (NPAR = 2 will
ensure both spin doublets)
state included (NPAR = 2 will
ensure both spin doublets)
ISYMSG: if ISYM =+1, then the electronic symmetry of the 2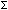 state
is sigma-plus
if ISYM = -1, then the electronic symmetry is sigma-minus
ISA: s/a symmetry index, if the molecule is homonuclear (ihomo=t)
then, if isa=+1 then only the s-levels (both for sigma and
pi) are included in the basis, if isa=-1, then only the
a-levels are included
state
is sigma-plus
if ISYM = -1, then the electronic symmetry is sigma-minus
ISA: s/a symmetry index, if the molecule is homonuclear (ihomo=t)
then, if isa=+1 then only the s-levels (both for sigma and
pi) are included in the basis, if isa=-1, then only the
a-levels are included
ISG: if isg=1 and ipi=0 then 2sig + atom scattering
IPI: if ipi=1 and isg=0 then 2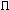 + atom
scattering
+ atom
scattering
if isg=1 and ipi=1 then 2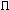 -2sig + atom scattering
-2sig + atom scattering
 Return to Hibridon Help
Return to Hibridon Help